Well-characterized safety and established management guidance1
After your patient receives their YESCARTA infusion, monitoring will take place at a healthcare facility, where there are approved safety protocols and physicians who are specially trained in YESCARTA administration and adverse event management. The healthcare facility will work with primary hematologists/oncologists to stay connected about treatment decisions, patient progress, and follow-up care. Though infusion and monitoring take place at the healthcare facility, long-term monitoring should continue in the community setting.1
The safety of YESCARTA in ≥3L relapsed/refractory (R/R) follicular lymphoma (FL) was evaluated in the ZUMA-5 pivotal trial.
Most cytokine release syndrome and neurologic toxicities in ZUMA-5 occurred early, were generally reversible, and were managed per established guidance.1,2
ZUMA-5 STUDY DESIGN1,3
- A phase 2, multicenter, single-arm, open-label study evaluating the efficacy and safety of YESCARTA in adults with R/R FL after ≥2 lines of therapy
- 146 patients received YESCARTA and were evaluated for safety
- 81 patients with FL were evaluated for efficacy
- Patients received lymphodepleting chemotherapy: 500 mg/m2 intravenous (IV) cyclophosphamide and 30 mg/m2 IV fludarabine (5, 4, and 3 days before infusion)
- YESCARTA was administered as a single infusion to a target dose of 2 x 106 viable CAR T cells/kg body weight
- Primary endpoint: ORR (CR + PR)
- Median age: 62 years (range: 34 to 79 years)
- Exclusion criteria: Active or serious infections, transformed lymphoma or other aggressive lymphomas, prior allogeneic H5CT, or any history of CNS lymphoma or CNS disorders
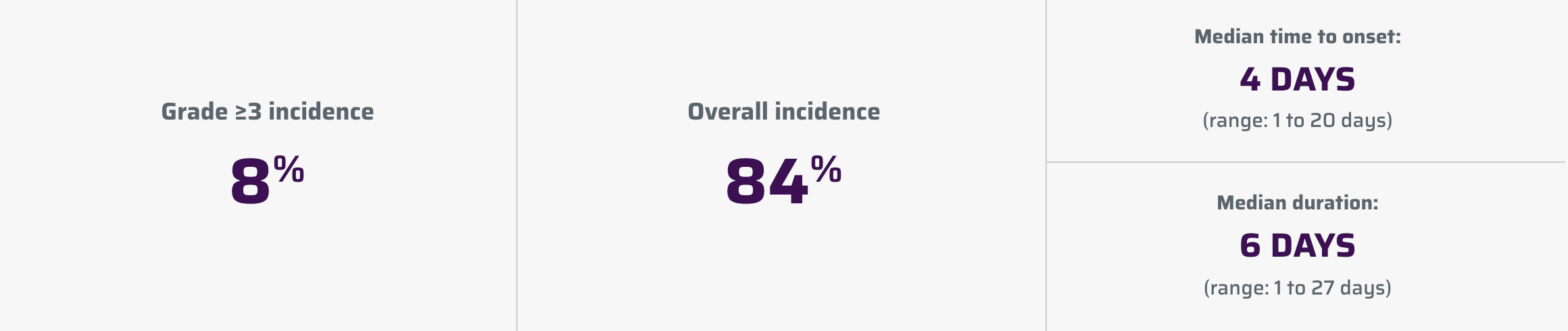
Cytokine release syndrome (CRS)1
CRS, including fatal or life-threatening reactions, occurred following treatment with YESCARTA.
Management of CRS1
Confirm that 2 doses of tocilizumab are available prior to infusion of YESCARTA. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time. At the first sign of CRS, treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids will be instituted as indicated. Identify CRS based on clinical presentation. Evaluate for and treat other causes of fever, hypoxia, and hypotension.
If CRS is suspected, manage according to the recommendations in Section 2.3 of the full Prescribing Information.
| CRS Grade | Tocilizumab | Corticosteroids |
|---|---|---|
| Grade 1 Symptoms require symptomatic treatment only (eg, fever, nausea, fatigue, headache, myalgia, malaise). | If symptoms (eg, fever) not improving after 24 hours, consider managing as Grade 2. | If not improving after 3 days, administer 1 dose of dexamethasone 10 mg intravenously. |
|
Grade 2 Symptoms require and respond to moderate intervention. Oxygen requirement less than 40% FiO2 or hypotension responsive to fluids or low-dose of 1 vasopressor or Grade 2 organ toxicity. |
Administer tocilizumab 8 mg/kg intravenously over 1 hour (not to exceed 800 mg). If no clinical improvement in the signs and symptoms of CRS after the first dose, repeat tocilizumab every 8 hours as needed. Limit to a maximum of 3 doses in a 24-hour period; maximum total of 4 doses. If improving, discontinue tocilizumab. |
Administer dexamethasone 10 mg intravenously once daily. If improving, manage as Grade 1 above and continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate. If not improving, manage as appropriate grade below. |
|
Grade 3 Symptoms require and respond to aggressive intervention. Oxygen requirement greater than or equal to 40% FiO2 or hypotension requiring high-dose or multiple vasopressors or Grade 3 organ toxicity or Grade 4 transaminitis. |
Per Grade 2. If improving, manage as appropriate grade above. |
Dexamethasone 10 mg intravenously 3 times a day. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate. If not improving, manage as Grade 4. |
|
Grade 4 Life-threatening symptoms. Requirements for ventilator support, continuous veno-venous hemodialysis (CVVHD) or Grade 4 organ toxicity (excluding transaminitis). |
Per Grade 2. If improving, manage as appropriate grade above. |
Administer methylprednisolone 1000 mg intravenously once per day for 3 days. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate. If not improving, consider methylprednisolone 1000 mg 2-3 times a day or alternate therapy.* |
*Alternate therapy includes (but is not limited to): anakinra, siltuximab, ruxolitinib, cyclophosphamide, IVIG, and ATG.1
Patients who experience Grade ≥2 CRS (eg, hypotension, not responsive to fluids, or hypoxia requiring supplemental oxygenation) should be monitored with continuous cardiac telemetry and pulse oximetry. For patients experiencing severe CRS, consider performing an echocardiogram to assess cardiac function. For severe or life-threatening CRS, consider intensive care supportive therapy.1
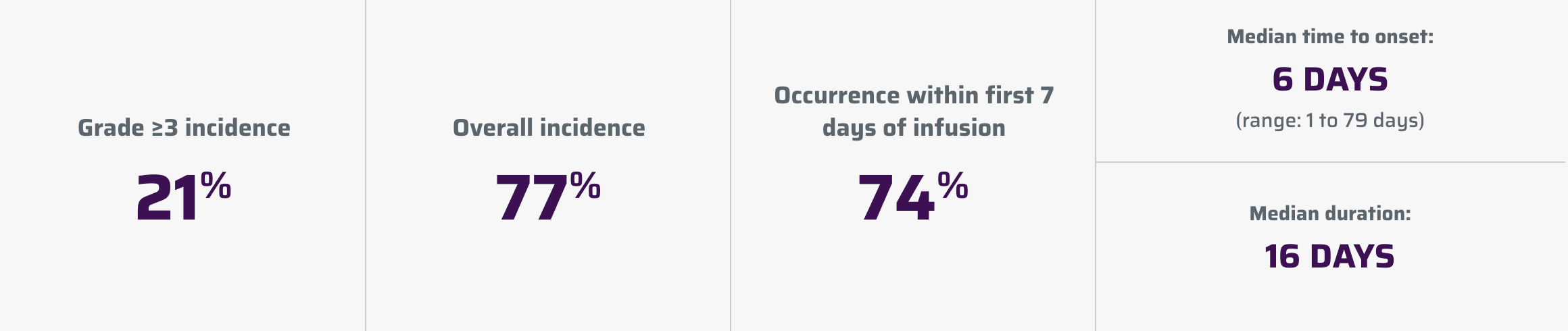
Neurologic toxicities1
Neurologic toxicities, which were fatal or life-threatening, occurred following treatment with YESCARTA.
Management of neurologic toxicities1
When managing neurologic toxicity/ICANS, rule out other causes of neurologic symptoms. Patients who experience Grade 2 or higher neurologic toxicities/ICANS should be monitored with continuous cardiac telemetry and pulse oximetry. Provide intensive-care supportive therapy for severe or life-threatening neurologic toxicities. Consider levetiracetam for seizure prophylaxis for any grade of neurologic toxicities.
See Section 2.3 of the full Prescribing Information for additional monitoring and management guidance relating to neurologic toxicities.
| Grading Assessment† | Concurrent CRS | No Concurrent CRS |
|---|---|---|
| Grade 1 |
Administer tocilizumab per CRS Grading and Management table above for management of Grade 1 CRS. In addition, administer 1 dose of dexamethasone 10 mg intravenously. If not improving after 2 days, repeat dexamethasone 10 mg intravenously. |
Administer 1 dose of dexamethasone 10 mg intravenously. If not improving after 2 days, repeat dexamethasone 10 mg intravenously. |
| Consider levetiracetam for seizure prophylaxis. | ||
| Grade 2 |
Administer tocilizumab per CRS Grading and Management table above for management of Grade 2 CRS. In addition, administer dexamethasone 10 mg intravenously 4 times a day. If improving, continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate. If not improving, manage as appropriate grade below. |
Administer dexamethasone 10 mg intravenously 4 times a day. If improving, continue corticosteroids until the severity is Grade 1 or less, then quickly taper as clinically appropriate. If not improving, manage as appropriate grade below. |
| Consider levetiracetam for seizure prophylaxis. | ||
| Grade 3 |
Administer tocilizumab per CRS Grading and Management table above for management of Grade 2 CRS. In addition, administer methylprednisolone 1000 mg intravenously once daily. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate. If not improving, manage as Grade 4. |
Administer methylprednisolone 1000 mg intravenously once daily. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate. If not improving, manage as Grade 4. |
| Consider levetiracetam for seizure prophylaxis. | ||
| Grade 4 |
Administer tocilizumab per CRS Grading and Management table above for management of Grade 2 CRS. In addition, administer methylprednisolone 1000 mg intravenously twice per day. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate. If not improving, consider 1000 mg of methylprednisolone intravenously 3 times a day or alternate therapy. |
Administer methylprednisolone 1000 mg intravenously twice per day. If improving, manage as appropriate grade above and continue corticosteroids until the severity is Grade 1 or less, then taper as clinically appropriate. If not improving, consider 1000 mg of methylprednisolone intravenously 3 times a day or alternate therapy.‡ |
| Consider levetiracetam for seizure prophylaxis. | ||
†Severity based on Common Terminology Criteria for Adverse Events.1
‡Alternate therapy includes (but is not limited to): anakinra, siltuximab, ruxolitinib, cyclophosphamide, IVIG, and ATG.1
Adverse reactions in ZUMA-51
The most common non-laboratory adverse reactions (incidence ≥20%) included fever, CRS, hypotension, encephalopathy, fatigue, headache, infections with pathogen unspecified, tachycardia, febrile neutropenia, musculoskeletal pain, nausea, tremor, chills, diarrhea, constipation, decreased appetite, cough, vomiting, hypoxia, arrhythmia, and dizziness.
Serious adverse reactions occurred in 48% of patients. Serious adverse reactions in >2% of patients included febrile neutropenia, encephalopathy, fever, CRS, infections with pathogen unspecified, pneumonia, hypoxia, and hypotension.
The most common (≥10%) Grade 3 or higher reactions included febrile neutropenia, encephalopathy, and infections with pathogen unspecified. Fatal adverse reactions occurred in 1% of patients and included CRS and fungal infection.
| Adverse Reaction | Any grade (%) | Grade ≥3 (%) |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Febrile neutropeniaa | 41 | 41 |
| Cardiac disorders | ||
| Tachycardiab | 44 | 1 |
| Arrhythmiac | 21 | 2 |
| Gastrointestinal disorders | ||
| Nausea | 40 | 0 |
| Diarrhead | 29 | 1 |
| Constipation | 28 | 0 |
| Vomiting | 24 | 1 |
| Abdominal paine | 16 | 0 |
| General disorders and administration site conditions | ||
| Fever | 85 | 8 |
| Fatiguef | 49 | 1 |
| Chills | 29 | 0 |
| Edemag | 13 | 1 |
| Immune system disorders | ||
| Cytokine release syndrome | 84 | 8 |
| Immunoglobulins decreasedh | 18 | 1 |
| Infections and infestations | ||
| Infections with pathogen unspecified | 45 | 14 |
| Pneumoniai | 13 | 8 |
| Fungal infections | 12 | 2 |
| Viral infections | 13 | 2 |
| Metabolism and nutrition disorders | ||
| Decreased appetitej | 26 | 1 |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal paink | 40 | 1 |
| Motor dysfunctionl | 18 | 2 |
| Nervous system disorders | ||
| Encephalopathym | 49 | 16 |
| Headache | 45 | 1 |
| Tremor | 31 | 1 |
| Dizzinessn | 20 | 0 |
| Aphasia | 14 | 4 |
| Neuropathy peripheralo | 12 | 0 |
| Ataxiap | 10 | 0 |
| Psychiatric disorders | ||
| Deliriumq | 16 | 5 |
| Insomnia | 16 | 0 |
| Affective disorderr | 10 | 1 |
| Respiratory, thoracic, and mediastinal disorders | ||
| Coughs | 25 | 0 |
| Hypoxia | 23 | 8 |
| Dyspneat | 12 | 1 |
| Nasal congestion | 10 | 0 |
| Skin and subcutaneous tissue disorders | ||
| Rashu | 19 | 3 |
| Vascular disorders | ||
| Hypotensionv | 51 | 4 |
| Hypertension | 13 | 6 |
| Thrombosisw | 12 | 4 |
aFebrile neutropenia includes febrile neutropenia, fever overlapping with neutropenia.
bTachycardia includes tachycardia, sinus tachycardia.
cArrhythmia includes atrial fibrillation, atrioventricular block first degree, bradycardia, sinus bradycardia, supraventricular tachycardia, ventricular arrhythmia, ventricular extra systoles, ventricular tachycardia, electrocardiogram QT prolonged, electrocardiogram T wave inversion.
dDiarrhea includes diarrhea, colitis, enteritis.
eAbdominal pain includes abdominal pain, abdominal discomfort, abdominal pain lower, abdominal pain upper, abdominal tenderness, dyspepsia, epigastric discomfort.
fFatigue includes asthenia, fatigue, decreased activity, malaise.
gEdema includes edema, face edema, generalized edema, localized edema, edema peripheral, peripheral swelling, pulmonary edema, swelling face.
hImmunoglobulins decreased includes hypogammaglobulinemia, blood immunoglobulin G decreased.
iPneumonia includes pneumonia streptococcal, pneumonia, lung infiltration. Pneumonia is also summarized under infections with pathogen unspecified.
jDecreased appetite includes decreased appetite, hypophagia.
kMusculoskeletal pain includes musculoskeletal pain, arthralgia, back pain, bone pain, flank pain, groin pain, musculoskeletal chest pain, myalgia, neck pain, osteoarthritis, pain in extremity.
lMotor dysfunction includes motor dysfunction, muscle rigidity, muscle spasms, muscle strain, muscular weakness.
mEncephalopathy includes agraphia, amnesia, aphonia, apraxia, CAR T-cell-related encephalopathy syndrome, cognitive disorder, disturbance in attention, dysarthria, dysgraphia, dyskinesia, encephalopathy, lethargy, loss of consciousness, memory impairment, somnolence, speech disorder, confusional state, mental status changes, immune effector cell-associated neurotoxicity, neurotoxicity, toxic encephalopathy.
nDizziness includes dizziness, presyncope, syncope, vertigo.
oNeuropathy peripheral includes allodynia, cervical radiculopathy, hyperesthesia, hypoesthesia, neuralgia, neuropathy peripheral, paresthesia, peripheral sensory neuropathy.
pAtaxia includes ataxia, balance disorder, gait disturbance, vestibular disorder.
qDelirium includes agitation, delirium, hallucination, restlessness.
rAffective disorder includes anxiety, depression, impulsive behavior, mania, panic attack.
sCough includes cough, productive cough, upper-airway cough syndrome.
tDyspnea includes dyspnea, dyspnea exertional.
uRash includes dermatitis bullous, erythema, pruritus, rash, rash macular, rash maculo-papular, Stevens-Johnson syndrome, urticaria.
vHypotension includes capillary leak syndrome, hypotension, hypoperfusion, orthostatic hypotension.
wThrombosis includes deep vein thrombosis, embolism, peripheral ischemia, pulmonary embolism, thrombosis in device, vascular occlusion, jugular vein thrombosis.
Patient monitoring1
Monitoring takes place at a healthcare facility where there are approved safety protocols and physicians who are specially trained in YESCARTA administration and adverse event management. Long-term monitoring should continue in the community setting.
7days
Patients will be monitored at least daily for 7 days following infusion for signs and symptoms of CRS and neurologic toxicities.
2weeks
Patients will be instructed to remain within proximity of a healthcare facility for at least 2 weeks following infusion. Monitor patients for signs or symptoms of CRS or neurologic toxicities for 2 weeks after infusion. Patients will be counseled to seek immediate medical attention should signs or symptoms of CRS or neurologic toxicities occur at any time.
3L=third line; ATG=anti-thymocyte globulin; CAR=chimeric antigen receptor; CNS=central nervous system; CR=complete remission; FiO2=fraction of inspired oxygen; HSCT=hematopoietic stem cell transplant; ICANS=immune effector cell-associated neurotoxicity syndrome; IVIG=intravenous immunoglobulin; ORR=objective response rate; PR=partial remission.
IMPORTANT SAFETY INFORMATION
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, and SECONDARY HEMATOLOGICAL MALIGNANCIES
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving YESCARTA. Do not administer YESCARTA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids.
- Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving YESCARTA, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with YESCARTA. Provide supportive care and/or corticosteroids as needed.
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA.
CYTOKINE RELEASE SYNDROME (CRS) CRS, including fatal or life-threatening reactions, occurred following treatment with YESCARTA. CRS occurred in 90% (379/422) of patients with non-Hodgkin lymphoma (NHL), including ≥ Grade 3 CRS in 9%. CRS occurred in 93% (256/276) of patients with large B-cell lymphoma (LBCL), including ≥ Grade 3 in 9%. Among patients with LBCL who died after receiving YESCARTA, 4 had ongoing CRS events at the time of death. For patients with LBCL in ZUMA-1, the median time to onset of CRS was 2 days following infusion (range: 1-12 days) and the median duration was 7 days (range: 2-58 days). For patients with LBCL in ZUMA-7, the median time to onset of CRS was 3 days following infusion (range: 1-10 days) and the median duration was 7 days (range: 2-43 days).
CRS occurred in 84% (123/146) of patients with indolent non-Hodgkin lymphoma (iNHL) in ZUMA-5, including ≥ Grade 3 CRS in 8%. Among patients with iNHL who died after receiving YESCARTA, 1 patient had an ongoing CRS event at the time of death. The median time to onset of CRS was 4 days (range: 1-20 days) and median duration was 6 days (range: 1-27 days) for patients with iNHL.
Key manifestations of CRS (≥ 10%) in all patients combined included fever (85%), hypotension (40%), tachycardia (32%), chills (22%), hypoxia (20%), headache (15%), and fatigue (12%). Serious events that may be associated with CRS include, cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), renal insufficiency, cardiac failure, respiratory failure, cardiac arrest, capillary leak syndrome, multi-organ failure, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS).
The impact of tocilizumab and/or corticosteroids on the incidence and severity of CRS was assessed in 2 subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received tocilizumab and/or corticosteroids for ongoing Grade 1 events, CRS occurred in 93% (38/41), including 2% (1/41) with Grade 3 CRS; no patients experienced a Grade 4 or 5 event. The median time to onset of CRS was 2 days (range: 1-8 days) and the median duration of CRS was 7 days (range: 2-16 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Thirty-one of the 39 patients (79%) developed CRS and were managed with tocilizumab and/or therapeutic doses of corticosteroids with no patients developing ≥ Grade 3 CRS. The median time to onset of CRS was 5 days (range: 1-15 days) and the median duration of CRS was 4 days (range: 1-10 days). Although there is no known mechanistic explanation, consider the risk and benefits of prophylactic corticosteroids in the context of pre-existing comorbidities for the individual patient and the potential for the risk of Grade 4 and prolonged neurologic toxicities.
Confirm that 2 doses of tocilizumab are available prior to infusion of YESCARTA. Monitor patients at least daily for 7 days following infusion for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for 2 weeks after infusion. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated.
NEUROLOGIC TOXICITIES Neurologic toxicities including immune effector cell-associated neurotoxicity syndrome (ICANS) that were fatal or life-threatening occurred following treatment with YESCARTA. Neurologic toxicities occurred in 78% (330/422) of patients with NHL receiving YESCARTA, including ≥ Grade 3 in 25%.
Neurologic toxicities occurred in 87% (94/108) of patients with LBCL in ZUMA-1, including ≥ Grade 3 in 31% and in 74% (124/168) of patients in ZUMA-7 including ≥ Grade 3 in 25%. The median time to onset was 4 days (range: 1-43 days) and the median duration was 17 days for patients with LBCL in ZUMA-1. The median time to onset for neurologic toxicity was 5 days (range: 1-133 days) and median duration was 15 days in patients with LBCL in ZUMA-7. Neurologic toxicities occurred in 77% (112/146) of patients with iNHL, including ≥ Grade 3 in 21%. The median time to onset was 6 days (range: 1-79 days) and the median duration was 16 days. Ninety-eight percent of all neurologic toxicities in patients with LBCL and 99% of all neurologic toxicities in patients with iNHL occurred within the first 8 weeks of YESCARTA infusion. Neurologic toxicities occurred within the first 7 days of infusion in 87% of affected patients with LBCL and 74% of affected patients with iNHL.
The most common neurologic toxicities (≥ 10%) in all patients combined included encephalopathy (50%), headache (43%), tremor (29%), dizziness (21%), aphasia (17%), delirium (15%), and insomnia (10%). Prolonged encephalopathy lasting up to 173 days was noted. Serious events, including aphasia, leukoencephalopathy, dysarthria, lethargy, and seizures occurred. Fatal and serious cases of cerebral edema and encephalopathy, including late-onset encephalopathy, have occurred.
The impact of tocilizumab and/or corticosteroids on the incidence and severity of neurologic toxicities was assessed in 2 subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received corticosteroids at the onset of Grade 1 toxicities, neurologic toxicities occurred in 78% (32/41) and 20% (8/41) had Grade 3 neurologic toxicities; no patients experienced a Grade 4 or 5 event. The median time to onset of neurologic toxicities was 6 days (range: 1-93 days) with a median duration of 8 days (range: 1-144 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Of those patients, 85% (33/39) developed neurologic toxicities; 8% (3/39) developed Grade 3 and 5% (2/39) developed Grade 4 neurologic toxicities. The median time to onset of neurologic toxicities was 6 days (range: 1-274 days) with a median duration of 12 days (range: 1-107 days). Prophylactic corticosteroids for management of CRS and neurologic toxicities may result in higher grade of neurologic toxicities or prolongation of neurologic toxicities, delay the onset, and decrease the duration of CRS.
Monitor patients for signs and symptoms of neurologic toxicities following infusion at least daily for 7 days; and for 2 weeks thereafter and treat promptly. Advise patients to avoid driving for at least 2 weeks following infusion.
HYPERSENSITIVITY REACTIONS Allergic reactions may occur with the infusion of YESCARTA. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO) or residual gentamicin in YESCARTA.
SERIOUS INFECTIONS Severe or life-threatening infections occurred after YESCARTA infusion. Infections (all grades) occurred in 45% of patients with NHL. Grade 3 or higher infections occurred in 17% of patients, including ≥ Grade 3 infections with an unspecified pathogen in 12%, bacterial infections in 5%, viral infections in 3%, and fungal infections in 1%. YESCARTA should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 36% of patients with NHL and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, including those who have received YESCARTA, life-threatening and fatal opportunistic infections including disseminated fungal infections (e.g., candida sepsis and aspergillus infections) and viral reactivation (e.g., human herpes virus-6 [HHV-6] encephalitis and JC virus progressive multifocal leukoencephalopathy [PML]) have been reported. The possibility of HHV-6 encephalitis and PML should be considered in immunosuppressed patients with neurologic events and appropriate diagnostic evaluations should be performed.
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, has occurred in patients treated with drugs directed against B cells, including YESCARTA. Perform screening for HBV, HCV, and HIV and management in accordance with clinical guidelines before collection of cells for manufacturing.
PROLONGED CYTOPENIAS Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and YESCARTA infusion. Grade 3 or higher cytopenias not resolved by Day 30 following YESCARTA infusion occurred in 39% of all patients with NHL and included neutropenia (33%), thrombocytopenia (13%), and anemia (8%). Monitor blood counts after infusion.
HYPOGAMMAGLOBULINEMIA B-cell aplasia and hypogammaglobulinemia can occur in patients receiving YESCARTA. Hypogammaglobulinemia was reported as an adverse reaction in 14% of all patients with NHL. Monitor immunoglobulin levels after treatment and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement.
The safety of immunization with live viral vaccines during or following YESCARTA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during YESCARTA treatment, and until immune recovery following treatment.
SECONDARY MALIGNANCIES Patients treated with YESCARTA may develop secondary malignancies. T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA. Mature T cell malignancies, including CAR-positive tumors, may present as soon as weeks following infusion, and may include fatal outcomes.
Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
ADVERSE REACTIONS The most common non-laboratory adverse reactions (incidence ≥ 20%) in patients with iNHL in ZUMA-5 included fever, CRS, hypotension, encephalopathy, fatigue, headache, infections with pathogen unspecified, tachycardia, febrile neutropenia, musculoskeletal pain, nausea, tremor, chills, diarrhea, constipation, decreased appetite, cough, vomiting, hypoxia, arrhythmia, and dizziness.
INDICATIONMORE
YESCARTA® is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy.
This indication is approved under accelerated approval based on response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trial(s).
Please see accompanying full Prescribing Information, including BOXED WARNING and Medication Guide.
Authorized Treatment Centers are independent facilities that dispense Kite CAR T therapies. Choice of an Authorized Treatment Center is within the sole discretion of the physician and patient. Kite does not endorse any individual treatment sites.
References: 1. YESCARTA® (axicabtagene ciloleucel). Prescribing information. Kite Pharma, Inc; 2025. 2. Neelapu SS, Chavez JC, Sehgal AR, et al. Three-year follow-up analysis of axicabtagene ciloleucel in relapsed/refractory indolent non-Hodgkin lymphoma (ZUMA-5). Blood. 2024;143(6):496-506. 3. Jacobson CA, Chavez JC, Sehgal AR, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91-103.