Choose their best chance at a cure* vs standard therapy1-4†
*Cure in LBCL can be defined as when the therapy has been stopped and (1) the survival curve plateaus; (2) 3-year OS data; and/or at least 2-year EFS data.3-6
Statistically significant improvement with YESCARTA1,7
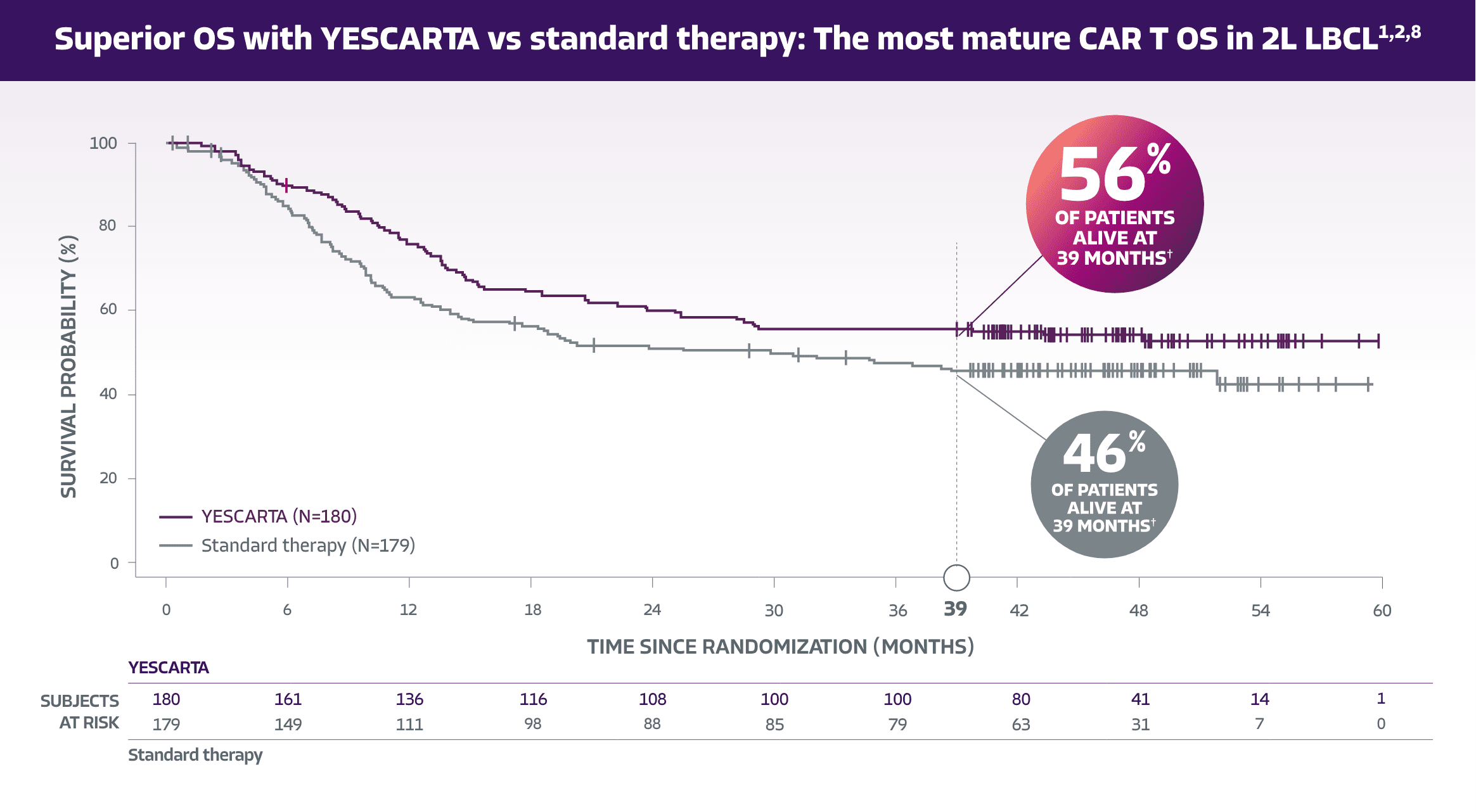
Median OS was NE with YESCARTA (95% CI: 28.6-NE) vs 31.1 months with ST (95% CI: 17.1-NE)1
Median OS was not estimable with YESCARTA at an estimated median follow-up of 46.7 months overall.1
†EFS is defined as the time from randomization to the earliest date of disease progression or relapse, best response of stable disease up to and including the day 150 assessment, commencement of new lymphoma therapy, or death from any cause. Response was assessed by an independent review committee, per the International Working Group Lugano classification (Cheson 2024).1
‡STUDY DESIGN: ZUMA-7 was a phase 3, randomized, open-label, multicenter study of YESCARTA single-infusion therapy vs standard therapy (salvage chemotherapy +/- HDT+ASCT) in 359 adult patients with R/R LBCL.1,7 Patients were randomized 1:1 to YESCARTA (N=180) and standard therapy (N=179) and stratified by response to 1L therapy and 2L age-adjusted IPI. The primary endpoint was EFS.1 The median follow-up time for the primary analysis was 22.1 months.1 Select secondary endpoints included OS, ORR, DOR, PFS, PROs, and safety.7,9,10
§Chemotherapy consisted of 2 or 3 cycles of chemoimmunotherapy.1
‖KM estimate.2
¶Not all data continued to be captured at the long-term follow-up. Types of data captured include investigator-assessed OS, PFS, and adverse events.8
#p-value is compared with 0.0249, the one-sided efficacy boundary (significance level) for the primary OS analysis.1
1L=first line; 2L=second line; ASCT=autologous stem cell transplant; CAR T=chimeric antigen receptor T cell; CI=confidence interval; DOR=duration of response; EFS=event-free survival; HDT=high-dose therapy; HR=hazard ratio; IPI=International Prognostic Index; KM=Kaplan-Meier; LBCL=large B-cell lymphoma; NE=not estimable; ORR=overall response rate; OS=overall survival; PFS=progression-free survival; PROs=patient-reported outcomes; R/R=relapsed/refractory.
IMPORTANT SAFETY INFORMATION
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, and SECONDARY HEMATOLOGICAL MALIGNANCIES
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving YESCARTA. Do not administer YESCARTA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids.
- Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving YESCARTA, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with YESCARTA. Provide supportive care and/or corticosteroids as needed.
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA.
CYTOKINE RELEASE SYNDROME (CRS)
CRS, including fatal or life-threatening reactions, occurred following treatment with YESCARTA. CRS occurred in 90% (379/422) of patients with non-Hodgkin lymphoma (NHL), including ≥ Grade 3 CRS in 9%. CRS occurred in 93% (256/276) of patients with large B-cell
lymphoma (LBCL), including ≥ Grade 3 in 9%. Among patients with LBCL who died after receiving YESCARTA, 4 had ongoing CRS events at the time of death. For patients with LBCL in ZUMA-1, the median time to onset of CRS was 2 days
following infusion (range: 1-12 days) and the median duration was 7 days (range: 2-58 days). For patients with LBCL in ZUMA-7, the median time to onset of CRS was 3 days following infusion (range: 1-10 days) and the median
duration was 7 days (range: 2-43 days).
CRS occurred in 84% (123/146) of patients with indolent non-Hodgkin lymphoma (iNHL) in ZUMA-5, including ≥ Grade 3 CRS in 8%. Among patients with iNHL who died after receiving YESCARTA, 1 patient had an ongoing CRS event at the time of death. The median time to onset of CRS was 4 days (range: 1-20 days) and median duration was 6 days (range: 1-27 days) for patients with iNHL.
Key manifestations of CRS (≥ 10%) in all patients combined included fever (85%), hypotension (40%), tachycardia (32%), chills (22%), hypoxia (20%), headache (15%), and fatigue (12%). Serious events that may be associated with CRS include, cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), renal insufficiency, cardiac failure, respiratory failure, cardiac arrest, capillary leak syndrome, multi-organ failure, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS).
The impact of tocilizumab and/or corticosteroids on the incidence and severity of CRS was assessed in 2 subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received tocilizumab and/or corticosteroids for ongoing Grade 1 events, CRS occurred in 93% (38/41), including 2% (1/41) with Grade 3 CRS; no patients experienced a Grade 4 or 5 event. The median time to onset of CRS was 2 days (range: 1-8 days) and the median duration of CRS was 7 days (range: 2-16 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Thirty-one of the 39 patients (79%) developed CRS and were managed with tocilizumab and/or therapeutic doses of corticosteroids with no patients developing ≥ Grade 3 CRS. The median time to onset of CRS was 5 days (range: 1-15 days) and the median duration of CRS was 4 days (range: 1-10 days). Although there is no known mechanistic explanation, consider the risk and benefits of prophylactic corticosteroids in the context of pre-existing comorbidities for the individual patient and the potential for the risk of Grade 4 and prolonged neurologic toxicities.
Confirm that 2 doses of tocilizumab are available prior to infusion of YESCARTA. Monitor patients at least daily for 7 days following infusion for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for 2 weeks after infusion. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated.
NEUROLOGIC TOXICITIES
Neurologic toxicities including immune effector cell-associated neurotoxicity syndrome (ICANS) that were fatal or life-threatening occurred following treatment with YESCARTA. Neurologic toxicities occurred in 78% (330/422) of patients with NHL receiving YESCARTA, including ≥ Grade 3 in 25%.
Neurologic toxicities occurred in 87% (94/108) of patients with LBCL in ZUMA-1, including ≥ Grade 3 in 31% and in 74% (124/168) of patients in ZUMA-7 including ≥ Grade 3 in 25%. The median time to onset was 4 days (range: 1-43 days) and the median duration was 17 days for patients with LBCL in ZUMA-1. The median time to onset for neurologic toxicity was 5 days (range: 1-133 days) and median duration was 15 days in patients with LBCL in ZUMA-7. Neurologic toxicities occurred in 77% (112/146) of patients with iNHL, including ≥ Grade 3 in 21%. The median time to onset was 6 days (range: 1-79 days) and the median duration was 16 days. Ninety-eight percent of all neurologic toxicities in patients with LBCL and 99% of all neurologic toxicities in patients with iNHL occurred within the first 8 weeks of YESCARTA infusion. Neurologic toxicities occurred within the first 7 days of infusion in 87% of affected patients with LBCL and 74% of affected patients with iNHL.
The most common neurologic toxicities (≥ 10%) in all patients combined included encephalopathy (50%), headache (43%), tremor (29%), dizziness (21%), aphasia (17%), delirium (15%), and insomnia (10%). Prolonged encephalopathy lasting up to 173 days was noted. Serious events, including aphasia, leukoencephalopathy, dysarthria, lethargy, and seizures occurred. Fatal and serious cases of cerebral edema and encephalopathy, including late-onset encephalopathy, have occurred.
The impact of tocilizumab and/or corticosteroids on the incidence and severity of neurologic toxicities was assessed in 2 subsequent cohorts of LBCL patients in ZUMA-1. Among patients who received corticosteroids at the onset of Grade 1 toxicities, neurologic toxicities occurred in 78% (32/41) and 20% (8/41) had Grade 3 neurologic toxicities; no patients experienced a Grade 4 or 5 event. The median time to onset of neurologic toxicities was 6 days (range: 1-93 days) with a median duration of 8 days (range: 1-144 days). Prophylactic treatment with corticosteroids was administered to a cohort of 39 patients for 3 days beginning on the day of infusion of YESCARTA. Of those patients, 85% (33/39) developed neurologic toxicities; 8% (3/39) developed Grade 3 and 5% (2/39) developed Grade 4 neurologic toxicities. The median time to onset of neurologic toxicities was 6 days (range: 1-274 days) with a median duration of 12 days (range: 1-107 days). Prophylactic corticosteroids for management of CRS and neurologic toxicities may result in higher grade of neurologic toxicities or prolongation of neurologic toxicities, delay the onset, and decrease the duration of CRS.
Monitor patients for signs and symptoms of neurologic toxicities following infusion at least daily for 7 days; and for 2 weeks thereafter and treat promptly. Advise patients to avoid driving for at least 2 weeks following infusion.
HYPERSENSITIVITY REACTIONS
Allergic reactions may occur with the infusion of YESCARTA. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO) or residual gentamicin in YESCARTA.
SERIOUS INFECTIONS
Severe or life-threatening infections occurred after YESCARTA infusion. Infections (all grades) occurred in 45% of patients with NHL. Grade 3 or higher infections occurred in 17% of patients, including ≥ Grade 3 infections with an unspecified pathogen in 12%,
bacterial infections in 5%, viral infections in 3%, and fungal infections in 1%. YESCARTA should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of
infection before and after infusion and treat appropriately. Administer prophylactic antimicrobials according to local guidelines.
Febrile neutropenia was observed in 36% of patients with NHL and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
In immunosuppressed patients, including those who have received YESCARTA, life-threatening and fatal opportunistic infections including disseminated fungal infections (e.g., candida sepsis and aspergillus infections) and viral reactivation (e.g., human herpes virus-6 [HHV-6] encephalitis and JC virus progressive multifocal leukoencephalopathy [PML]) have been reported. The possibility of HHV-6 encephalitis and PML should be considered in immunosuppressed patients with neurologic events and appropriate diagnostic evaluations should be performed.
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, has occurred in patients treated with drugs directed against B cells, including YESCARTA. Perform screening for HBV, HCV, and HIV and management in accordance with clinical guidelines before collection of cells for manufacturing.
PROLONGED CYTOPENIAS
Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and YESCARTA infusion. Grade 3 or higher cytopenias not resolved by Day 30 following YESCARTA infusion occurred in 39% of all patients with NHL and
included neutropenia (33%), thrombocytopenia (13%), and anemia (8%). Monitor blood counts after infusion.
HYPOGAMMAGLOBULINEMIA
B-cell aplasia and hypogammaglobulinemia can occur in patients receiving YESCARTA. Hypogammaglobulinemia was reported as an adverse reaction in 14% of all patients with NHL. Monitor immunoglobulin levels after treatment and manage using infection precautions,
antibiotic prophylaxis, and immunoglobulin replacement.
The safety of immunization with live viral vaccines during or following YESCARTA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during YESCARTA treatment, and until immune recovery following treatment.
SECONDARY MALIGNANCIES
Patients treated with YESCARTA may develop secondary malignancies. T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies, including YESCARTA. Mature T cell malignancies, including CAR-positive tumors, may present as soon as weeks following infusion, and may include fatal outcomes.
Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 20%) in:
- patients with LBCL in ZUMA-7 included fever, CRS, fatigue, hypotension, encephalopathy, tachycardia, diarrhea, headache, musculoskeletal pain, nausea, febrile neutropenia, chills, cough, infection with unspecified pathogen, dizziness, tremor, decreased appetite, edema, hypoxia, abdominal pain, aphasia, constipation, and vomiting.
- patients with LBCL in ZUMA-1 included CRS, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, decreased appetite, chills, diarrhea, febrile neutropenia, infections with pathogen unspecified, nausea, hypoxia, tremor, cough, vomiting, dizziness, constipation, and cardiac arrhythmias.
INDICATIONSMORE
YESCARTA® is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of:
- Adult patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy.
- Adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
Limitations of Use: YESCARTA is not indicated for the treatment of patients with primary central nervous system lymphoma.
Please see full Prescribing Information, including BOXED WARNING and Medication Guide.
Authorized Treatment Centers are independent facilities that dispense Kite CAR T therapies. Choice of an Authorized Treatment Center is within the sole discretion of the physician and patient. Kite does not endorse any individual treatment sites.
References: 1. YESCARTA® (axicabtagene ciloleucel). Prescribing information. Kite Pharma, Inc; 2025. 2. Westin JR, Oluwole OO, Kersten MJ, et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N Engl J Med. 2023;389(2):148-157 (suppl). doi:10.1056/NEJMoa2301665 3. Ravi P, Kumar SK, Cerhan JR, et al. Defining cure in multiple myeloma: a comparative study of outcomes of young individuals with myeloma and curable hematologic malignancies. Blood Cancer J. 2018;8(3):26. doi:10.1038/s41408-018-0065-8 4. Patel AR, Ray M, Rodriguez-Guadarrama YA, et al. Statistical challenges from trials of potentially curative treatments: validation of cure assumptions when analyzing ZUMA-7 follow-up data of axi-cel and standard of care therapy. Blood. 2023;142 (suppl 1):6899. doi:10.1182/blood-2023-181255 5. Howlader N, Mariotto AB, Besson C, et al. Cancer-specific mortality, cure fraction, and noncancer causes of death among diffuse large B-cell lymphoma patients in the immunochemotherapy era. Cancer. 2017;123(17):3326-3334. 6. Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066-1073. 7. Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-654. 8. Westin JR, Oluwole OO, Kersten MJ, et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N Engl J Med. 2023;389(2):148-157. 9. Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-654 (protocol). doi:10.1056/NEJMoa2116133 10. Elsawy M, Chavez JC, Avivi I, et al. Patient-reported outcomes in ZUMA-7, a phase 3 study of axicabtagene ciloleucel in second-line large B-cell lymphoma. Blood. 2022;140(21):2248-2260. 11. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed February 17, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org.





